Alexandre-Emile Béguyer de Chancourtois

De Chancourtois was born in Paris. At age eighteen, he entered the renowned École Polytechnique, one of the best known French grandes Écoles of engineering and management. After completing his studies at École Polytechnique, de Chancourtois went on a geological expedition into Hungary, Armenia and Turkey. In 1848, de Chancourtois went back to Paris and joined the teaching faculty as professor of mine surveying at the école Nationale Supérieure des Mines de Paris.
De Chancourtois led several overseas expeditions during the course of his life and served as the Inspector of Mines in Paris from 1875 until his death. As a mine inspector, he introduced safety laws to prevent methane gas explosions, which were frequent occurrences at the time.
All Periodic Tables list the elements in order of a particular property. A property which can be expressed by a number (such as relative atomic mass) is better than a property which cannot (such as color). In 1860 a conference was held in Karlsruhe (Germany) which produced a much more accurate list of atomic weights than previously available. (Not only were some earlier values slightly inaccurate, faulty reasoning had led to some being a half or a third of the correct value).

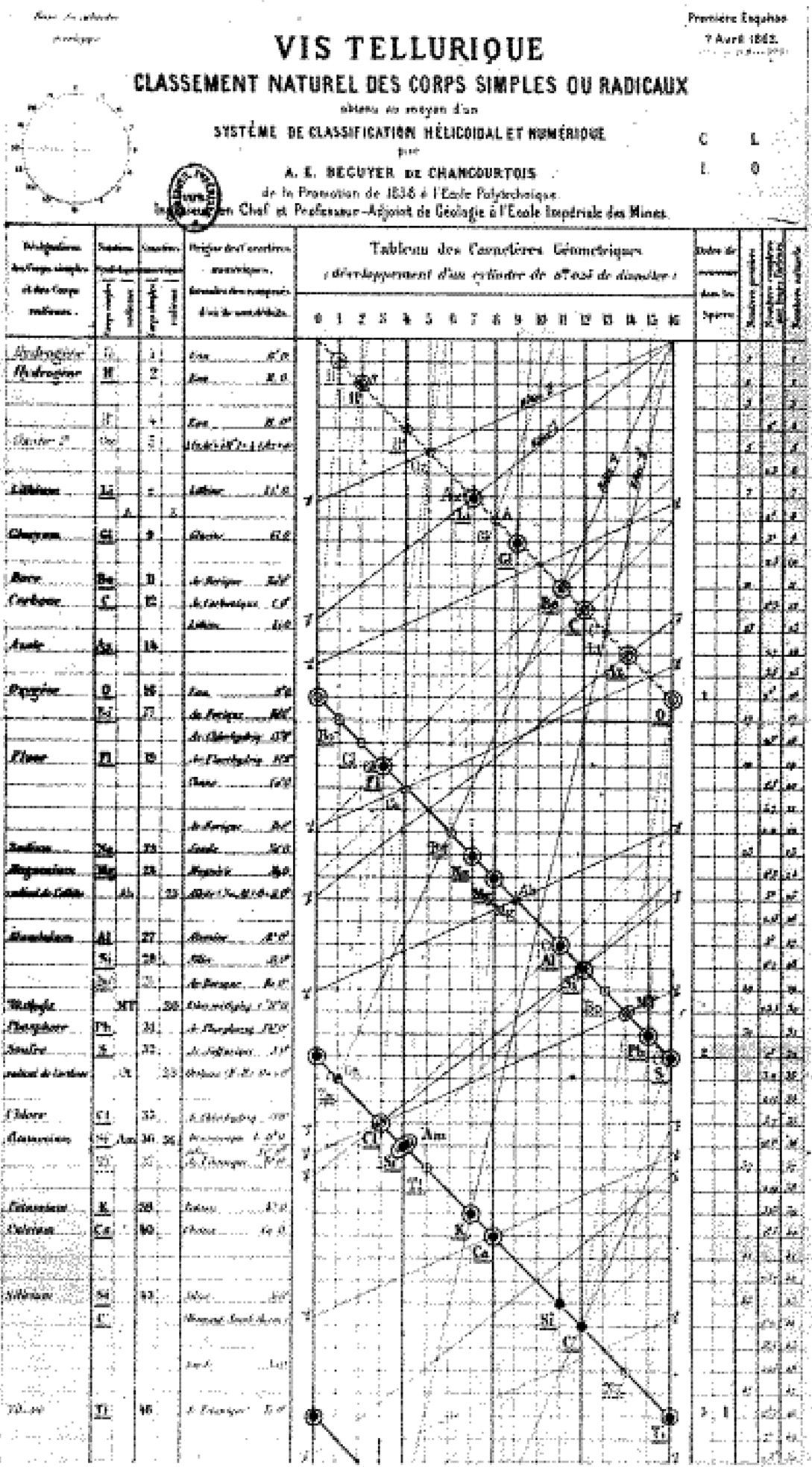
In 1862, a year before John Alexander Reina Newlands published his classification of the elements, the Law of Octaves, and about 7 years prior to Mendeleev's Periodic System development, de Chancourtois created a fully-functioning and unique system of organizing the chemical elements. He devised a spiral graph that was arranged on a cylinder which he called Vis Tellurique, seen in the wooden case, above.
De Chancourtois ordered the elements by increasing atomic weight and with similar elements lined up vertically, and with all the elements in an unbroken order. He plotted the equivalent weights on the surface of a cylinder with a circumference of 16 units, the approximate atomic weight of oxygen.
The resulting 3D spiral chart had elements with like properties above or below one another on the cylinder as well. He suggested that "The properties (of elements) are the properties of numbers", and was the first scientist to recognize that similar elements occurred at regular intervals - the basic principle of all succeeding periodic tables.
source: RSC.org, Wikipedia
< BACK
![]()
![]()